Starch, glycogen, cellulose, and chitin are four of the most common substances in nature… and guess what? They are all composed of thousands of glucose molecules bonded together with glycosidic bonds! In other words, they are all polysaccharides (complex carbs) that just use glucose over and over.
Think back to kindergarden when you and the other peewees built things out of blocks. To build starch, glycogen, cellulose, and chitin, you’d just use one type of block, always the same color and always the same size. However, you could put multiple copies of that same block together in different ways to form different polysaccharides.
Other polysaccharides contain other simple sugars besides glucose as building blocks, or even a mixture of more than one type of simple sugar, but they are less common than these four extremely abundant substances made of all glucose units.

- Starch
Starch is the way plants store extra glucose they don’t immediately use so it can be used as energy in the future when they need it. When we eat a potato or a piece of bread full of plant starch, our bodies break down that starch, a polymer consisting of thousands of glucose molecules, into the basic building blocks of glucose. We can then use those glucose molecules for immediate energy or convert them into another form of glucose storage, glycogen. Or if our metabolism doesn’t work properly (for example, we have type 2 diabetes) we easily convert that glucose into fat.
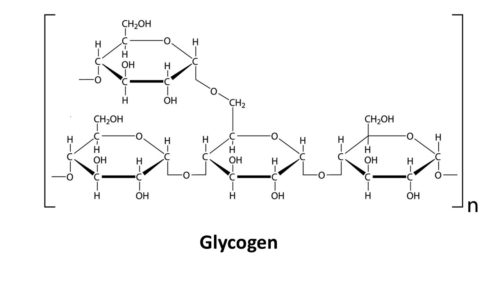
- Glycogen
While plants store glucose in the form of starch, animals, including humans, store glucose in the form of glycogen. Sometimes glycogen is referred to as “animal starch” because plant starch and glycogen are so similar in structure and both are used to store extra glucose, i.e. they are “storage polysaccharides.” Like starch, glycogen is made from thousands of glucose molecules all bonded together. We store glycogen primarily in our liver and muscle cells. However, we can also store a little glycogen in our kidneys and the glial cells in our brain (glial cells protect our neurons and help them communicate). Omnivores also eat glycogen when they eat meat since it is stored in the muscle tissues of other animals. The body breaks glycogen down much like it breaks down plant starch.
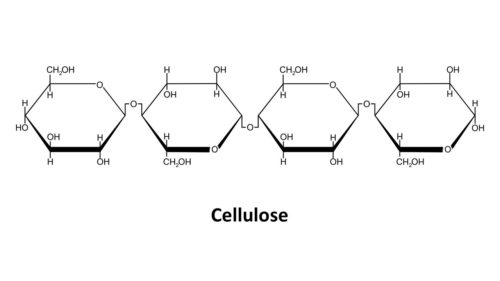
- Cellulose
Some polysaccharides are used to form rigid structures. Cellulose, also formed by bonding together thousands of glucose molecules, is used to form the cell walls of plants and algae. It’s also produced by bacteria when they form biofilms. It’s what makes redwood trees stand up tall and sturdy and what makes cotton and hemp such great sturdy fibers to make clothing from. Paper would not exist without cellulose! Humans do not have the enzyme necessary to digest cellulose so we excrete this in our stools. For us, cellulose is insoluble fiber.
In fact, the cell walls in some plant and algae are so thick and tough we can’t get to the nutrients inside through mechanical digestion alone. This is why health foods like chlorella – green algae sold in powder form and often added to smoothies – has to have the cell wall cracked open before we eat it. Otherwise, the whole little algae cell would pass through completely undigested.
In fact, the cell walls in some plant and algae are so thick and tough we can’t get to the nutrients inside through mechanical digestion alone. This is why health foods like chlorella – green algae sold in powder form and often added to smoothies – has to have the cell wall cracked open before we eat it. Otherwise, the whole little algae cell would pass through completely undigested.
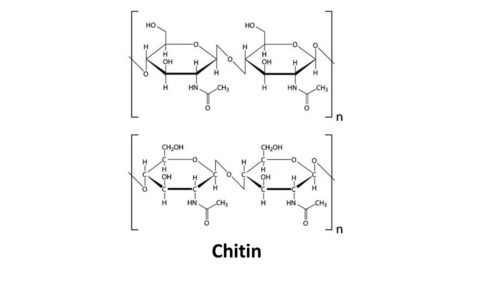
- Chitin
Chitin is another very common glucose-based polysaccharide. It forms the hard exoskeleton of all insects and crustaceans like shrimp and crab. It also forms the cell wall of mushrooms and the beaks of squid and octopi. For a long time, scientists thought that chitin was completely undigestible to humans.
However, in 2007, a group of scientists from the University of Padova in Italy discovered that a group of enzymes, called chitinases, capable of breaking down chiton, do indeed occur in the human intestine! It is not clear yet whether this means we can tap into some of the glucose stored in chitin as food.
However, it is definitely thought that these enzymes play a role in warding off intestinal parasites as it allows us to break them down their exoskeletons! The researchers involved in the study suggested that chitinases may be missing in some human populations that do not eat foods containing chiton.
Okay, as you can see, the basic units of glucose can combine together to form several different substances, including one of our most common food sources (starch) and our way of storing glucose energy for later; either in the form of glycogen or fat.
If you look at these schematic diagrams of starch, glycogen, cellulose, and chitin, it may make your mind spin! The structures get really complicated because glucose doesn’t just form straight lines when it connects together to form polysaccharides. As these polysaccharides form, side branches are also formed. Also, these side branches can branch off too! Additionally, straight lines of glucose molecules can fold up or twist like spirals.
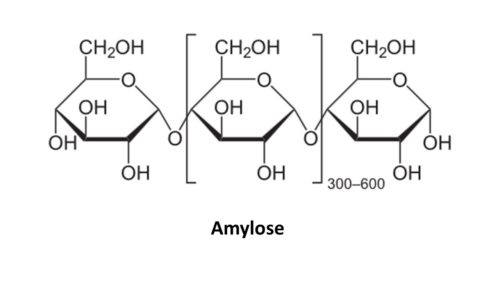
More about starch
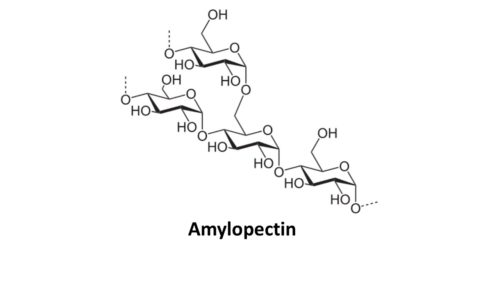
Starch is actually composed of two types of glucose chains. One is called amylose and the other is called amylopectin. Amylose is simpler in structure than amylopectin. Amylose is basically straight chains of glucose rings where the #1 carbon on one glucose ring connects to the #4 carbon on the next glucose ring, and so on. These are called 1,4 glycosidic bonds, with the numbers referring to the specific position of the carbons in the glucose rings. These straight chains of glucose rings, however, do not remain straight in amylose. Instead, they twist to form a helical structure. Amylopectin also has 1,4 glycosidic bonds and coils too. However, it also creates side branches by connecting the #1 carbon on one glucose molecule to the #6 carbon on another glucose molecule.
Glycogen is very similar to the amylopectin portion of starch, only the side branches are much closer together.
Cellulose is more like amylose with 1,4 glycosidic bonds, forming unbranched chains of glucose rings. However, the structure of half of the individual glucose molecules are in a “beta” position rather than an “alpha” position. This has to do with how the hydroxyl groups (-OH), an oxygen atom connected to a hydrogen atom, are positioned on the glucose ring. Basically, alpha glucose and beta glucose are mirror images of one another but they have the same ring structure and the same number of carbons, oxygens, and hydrogens.
The effect of having alternating alpha position glucose molecules and beta position glucose molecules in the chain of glucose molecules makes them NOT form a helix like they do the amylose portion of starch. Instead, they lay flat, making them perfect for building multi-layered cell walls in plants. Chitin is a lot like cellulose only an acetyl amine group (contains nitrogen) is substituted for one of the hydroxyl group (-OH). This one simple change gives chitin more strength and flexibility than cellulose.

Leave a Reply
You must be logged in to post a comment.